Zirconia, also known as zirconium dioxide (Zr02), is found in its most natural form in the mineral baddeleyite. But it can also be chemically derived from zircon. It is the most commercially important oxide formed by zircon.
Zirconia products are characterised by good mechanical properties and stability at high temperatures, strong thermal and corrosion resistance, chemical inertness and consistent quality. This makes them ideal for use in a wide range of refractory products, ceramic colours and pigments and electronic applications.
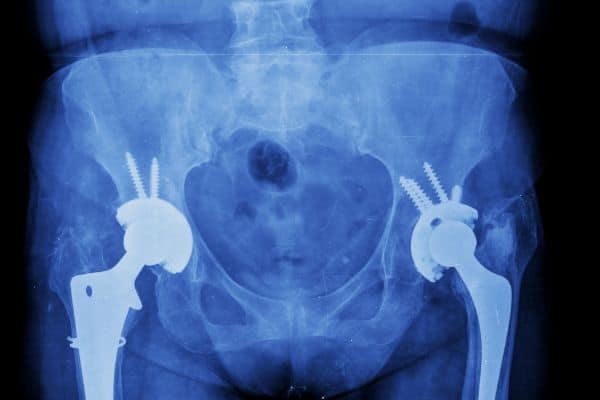
Other applications include friction materials, welding rods and zirconium metal and alloys. Advanced zirconia ceramics have excellent biocompatibility ensuring zirconia has replaced alumina as the material of choice for prosthesis devices such as hip joints or femoral ball heads. It has superior strength and hardness, wear resistance, stability, resistance to scratching and biocompatibility with the human body. One of zirconia’s other most common uses is within dental implants.
Cubic zirconia is one of the most well-known forms of zirconia. Commonly known as a synthetic diamond, it has become popular as a gemstone due to its optically clear single crystals and high refractive index, as well as its ability to maintain its colour and brilliance.
Zirconia, also known as zirconium dioxide (Zr02), is found in its most natural form in the mineral baddeleyite. But it can also be chemically derived from zircon. It is the most commercially important oxide formed by zircon.
The methods used to obtain zirconia from zircon, although distinct from each other, have three common features:

A number of methods are used to obtain zirconia from zircon.
The principal methods for commercial production of zirconia include:
Fused zirconia (zirconium oxide) is produced through the reduction and fusion of zircon sand (zirconium silicate). Zircon is mixed with coke and heated to its fusion point (in excess of 2,800 ̊C) in an electric arc furnace where it dissociates to zirconium oxide and fumed silica.

Zircon is mixed with coke and heated to its fusion point (in excess of 2,800 ̊C) in an electric arc furnace where it dissociates to zirconium oxide and fumed silica.
Zircon, also referred to as zirconium silicate (ZrSiO4), is a co-product from the harvesting and processing of ancient heavy mineral sand deposits. Found mainly in Australia and South Africa, zircon can be used either in its coarse sand form or milled to a fine powder. Its properties ensure that it is used in many everyday products, including ceramic tiles and medical implants, as well as having major industrial applications.
Zircon can be processed to create zirconia by melting the sand at very high temperatures to form molten zirconia, also known as zirconium oxide (ZrO2).
Zirconium, another derivative of zircon, is the chemical element Zr in the Periodic Table and takes the form of a silvery grey metal. As the 18th most abundant element in the earth’s crust, it commonly occurs in the mineral zircon in silicate form and, less frequently, in the mineral baddeleyite in oxide form.
Zircon has a theoretical content of 67% zirconia and 32% silica and it can typically contain a small percentage of hafnium in the range of 0.2 to 4%.
Zircon sand (zirconium silicate) is mined from mineral sand deposits.
Used mainly in the ceramics industry, zircon flour is manufactured by milling zircon sand.
Find out how zircon, used as an opacifier, provides high whiteness and brightness in ceramics.
There is a wide range of zirconium-based chemicals.
Zirconia, found naturally in baddeleyite, is also chemically derived from zirconium.
Zirconium, a silvery grey metal, is the chemical element Zr in the Periodic Table.
Zirconium sponge is used mainly in the nuclear industry.
A summary of the unique physical properties of zircon.