Zircon, also referred to as zirconium silicate (ZrSiO4), is a co-product from the harvesting and processing of ancient heavy mineral sand deposits. Found mainly in Australia and South Africa, zircon can be used either in its coarse sand form or milled to a fine powder. Its properties ensure that it is used in many everyday products, including ceramic tiles and medical implants, as well as having major industrial applications.
Zircon can be processed to create zirconia by melting the sand at very high temperatures to form molten zirconia, also known as zirconium oxide (ZrO2).
Zirconium, another derivative of zircon, is the chemical element Zr in the Periodic Table and takes the form of a silvery grey metal. As the 18th most abundant element in the earth’s crust, it commonly occurs in the mineral zircon in silicate form and, less frequently, in the mineral baddeleyite in oxide form.
Zircon has a theoretical content of 67% zirconia and 32% silica and it can typically contain a small percentage of hafnium in the range of 0.2 to 4%.
Zircon sand (zirconium silicate) is mined from mineral sand deposits.
Used mainly in the ceramics industry, zircon flour is manufactured by milling zircon sand.
Find out how zircon, used as an opacifier, provides high whiteness and brightness in ceramics.
There is a wide range of zirconium-based chemicals.
Zirconia, found naturally in baddeleyite, is also chemically derived from zirconium.
Zirconium, a silvery grey metal, is the chemical element Zr in the Periodic Table.
Zirconium sponge is used mainly in the nuclear industry.
A summary of the unique physical properties of zircon.
Zircon, also known as zircon silicate (ZrSiO4), is found in ancient mineral sand deposits. In the form of crystal sands, zircon is typically brown, but could also vary from colourless to yellow-golden, pink and red to blue and green.
Deposits of mineral sands are formed along ancient coastlines where the heavier minerals were concentrated by wave and wind action. The majority of zircon sand is mined in Australia and the African continent, with current annual global production in excess of one million tonnes. Mining of mineral sands can be by both dry mining and wet (dredge) mining methods.
The main use of zircon sand is its conversion into flour, opacifier, fused zirconia, zirconium chemicals, chemical zirconia and zirconium metal. Zircon sand is directly used in foundry applications and refractories and other minor applications.

Zircon flour is manufactured by milling zircon sand, usually in ball mills, either in the dry state or in a slurry.
It is used in a variety of applications, including ceramic frits, foundry mould coatings, ceramic shells for investment casting, refractories, friction products, insulating fibres and glass.
Depending on the application, there are various specifications for particle size, typically -200 mesh (-75 µm) and -325 mesh (-45 µm).
Zircon flour is packaged in bulk bags or paper sacks, or delivered in tankers (as powder or slurry/slop).
Zircon flour is used in the ceramic glaze, glass, refractory and plastics industry. It is ideal for use as an opacifier for glaze frits. Small additions of zircon can help to improve the mechanical strength of glass and impart alkali resistance to the glass fibres used in glass reinforced concrete. Zircon flour can also be used as a refractory wash for glost kilns, as a fine constituent in pressed zircon refractory shapes and, as filler for epoxy resins. Finely-milled zircon, ‘zircon flour’, is also used in refractories and friction products.

The most important market for zircon by volume is the ceramics industry. Within this sector, the principal use of zircon is as an opacifier (or whitener) for the ceramic body and surface. As a fine powder, zircon can be used directly in ceramic mixtures to increase the whiteness of the entire ceramic body or used in engobes to produce a white and opaque layer that hides the colour of the body. It’s also used as a raw material in ceramic glazes to increase their opacity, or in frit compositions used to produce glossy, opaque, white glazes. When used in surface glazes, zircon significantly enhances resistance to abrasion and chemical attack.
The properties that make zircon a suitable opacifier are its high refractive index. Finely milled zircon grains scatter visible light, making ceramics appear white, glossy and opaque.
There are various grades of zircon opacifier, including -10 µm, -5 µm and -1 µm products with D50 ranging from 2 µm down to 1µm.

There is a wide range of zirconium-based chemicals, used either for their intrinsic properties in diverse applications, or as intermediates for the manufacture of other derivatives of zirconium. Zirconium chemicals include:

Zirconia, also known as zirconium dioxide (Zr02), is found in its most natural form in the mineral baddeleyite. But it can also be chemically derived from zircon. It is the most commercially important oxide formed by zircon.
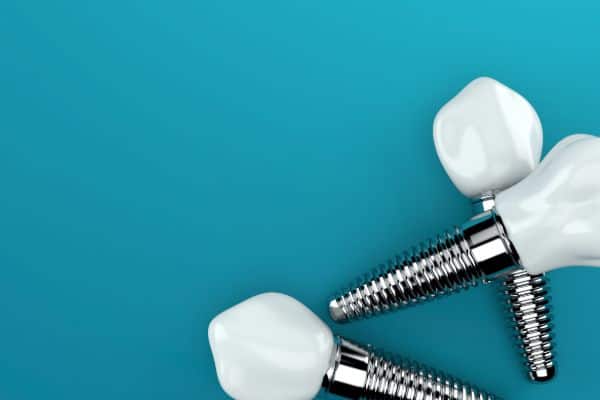
Zirconia products are characterised by good mechanical properties and stability at high temperatures, strong thermal and corrosion resistance, chemical inertness and consistent quality. It is used in a vast array of applications from refractory products, ceramic colours, and electronic applications to medial prosthesis devices such as hip joints and dental implants.
Zirconium, a derivative of zircon, is the chemical element Zr in the Periodic Table and takes the form of a silvery grey metal. It is the 20th century’s most abundant element in the earth’s crust and commonly occurs in the mineral zircon, in silicate form and less frequently in the mineral baddeleyite in oxide form.
Around 97% of zirconium compounds and zirconium metal produced worldwide are obtained from zircon recovered from heavy minerals sands deposits, while the rest is obtained from primary igneous deposits such as baddeleyite mining. Zirconium is typically produced by the reduction of the chemical zirconium oxychloride (ZOC), where the ZOC itself is produced by a complex process of chemical disassociation of zircon (zirconium silicate).
Used mainly as an alloy in the nuclear power industry, zirconium can also be added to aluminium alloys and steel to improve mechanical properties and corrosion resistance. Zirconium chemicals are used in a vast array of applications from catalysts to paper coatings and cosmetics.

Zirconium sponges are produced by injecting gas or mixing a foaming agent into molten metal, creating a froth that is stabilised by a high-temperature foaming agent. Used mainly in the nuclear industry, it is also used to produce zirconium alloys for nuclear reactor components, such as cladding for fuel rods.
Zirconium sponges are also used in a wide variety of applications including heat exchangers, for energy absorption, flow diffusion, and lightweight optics.

Zircon (zirconium silicate) has a unique set of physical properties which make it suitable for use in a variety of demanding applications.
These properties are summarised below.
| PROPERTY | APPLICATION |
|---|---|
| High refractive index | Ceramics (zircon acts as an opacifier and enhances whiteness) |
| High hardness | Ceramics (zircon gives resistance to scratching and mechanical damage) |
| High melting point | Refractory industry (and by extension the metal casting industry) |
|
High spatial and thermal stability at elevated temperatures Low coefficient of linear expansion - good resistance to thermal shock Moderate to high thermal conductivity Low wettability by molten metal Clean and round grains, which can be bonded at little cost and with little material Binding ability with all organic and inorganic moulding sand binders |
Metal casting and refractory industries
|
| Chemical stabillty | Many applications, including metal casting |
| Low solubility in molten silica or silicates | Glass refractories |
| Good dielectric properties | Technical and advanced ceramics |